Atomic Structure and Bonding
Now we get to chat about the little things than make up all things: atoms.
Atoms are single units that combine and react to make all matter.
Atomic Theory
Atoms are made of three units: Protons, Neutrons and Electrons.
Atoms have a central nucleus and shells, or “energy levels”, of electrons surrounding it.
The nucleus contains protons and neutrons. Electrons orbit the nucleus.
Different elements have different numbers of protons. Hydrogen has 1 proton, Carbon has 6 protons, Chlorine has 17 protons. There are an equal number of electrons as protons.
The number of protons in an atom is called the Atomic Number.
The number of protons + the number of neutrons is the Mass Number.
Protons have a mass of 1, neutrons have a mass of 1, electrons have a mass of 0.
Protons have a charge of 1+, neutrons have a charge of 0, electrons have a charge of 1-.
The Periodic Table
The elements in the periodic table are arranged in order of increasing atomic number. The vertical columns are called groups and the horizontal rows are called periods.
Elements in the same group have the same number of electrons in their outer energy level.
Elements in the same period have the same number of energy levels.
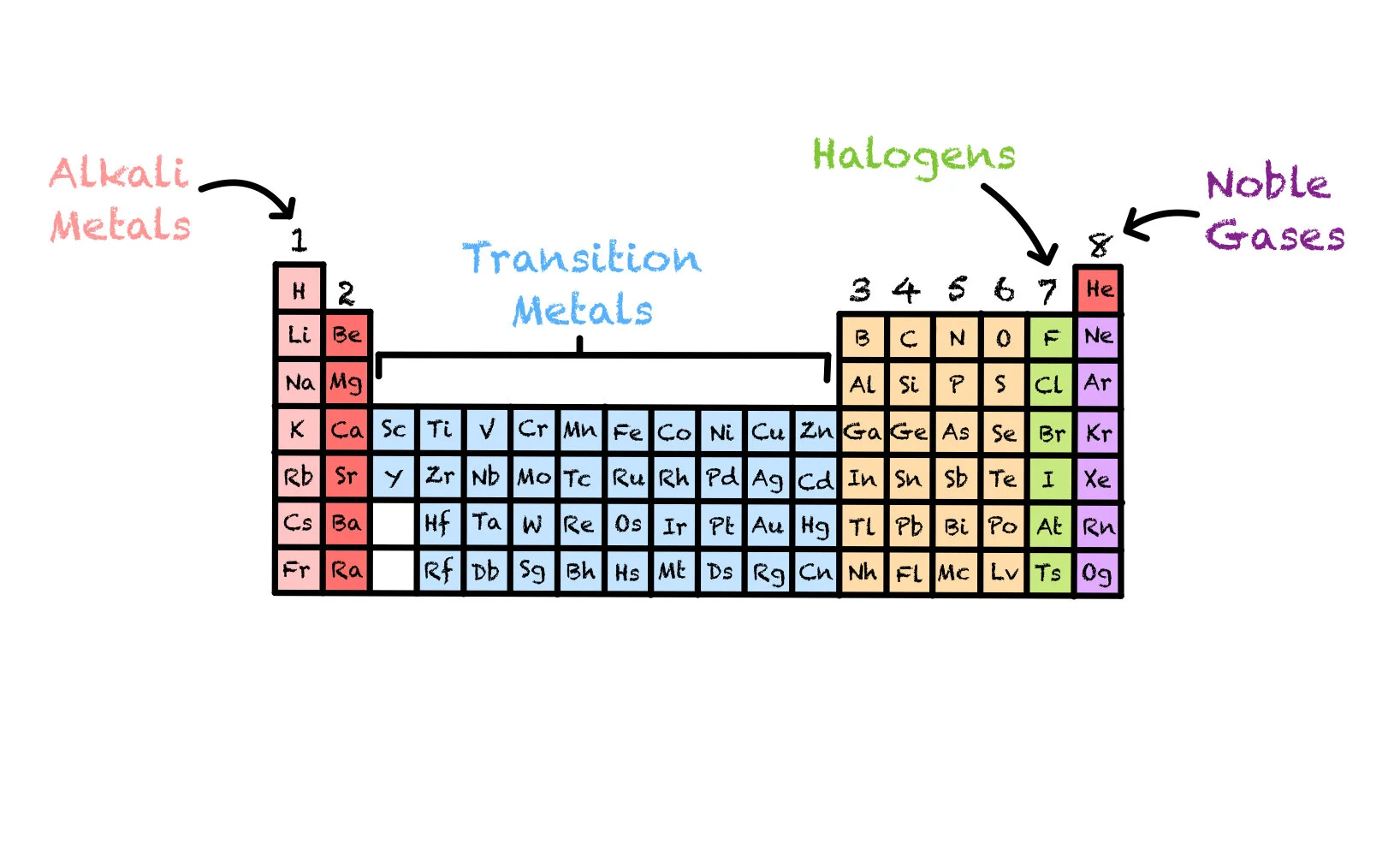
You need to be familiar with groups 1, 7, 8 and the transition metals.
Group 1 are the Alkali Metals. These are very reactive as they have only one outer electron.
The transition metals are the block of elements in the middle of the periodic table.
Group 7 are the Halogens. These are very reactive as they only require one electron to have a full outer energy level.
Group 8 are the Noble Gases. These are not reactive as they have a full, stable outer shell of electrons.
Valency and Ions
Valency is an element’s capacity to combine with other elements. The valency of an element is determined by the number of electrons in an atom’s outer energy level, and so elements in the same group have the same valency.
Group 8 has a valency of 0.
Groups 1 and 7 have a valency of 1.
Groups 2 and 6 have a valency of 2.
Groups 3 and 5 have a valency of 3.
Group 4 has a valency of 4.
An ion is a particle, atom or molecule with a net electrical charge. This may be positive if there are more protons than electrons, or negative if there are more electrons than protons.
So if you added an electron to Fluorine, it would become a negative ion with charge 1-. If you took an electron away from Fluorine, it would become a positive ion with charge 1+.
Nuclide Notation and Isotopes
Isotopes are atoms that have the same number of protons but a different number of neutrons. The number of neutrons within the atoms of a particular element can therefore vary. The relative atomic mass of an elements is the average weighted mass of the isotopes of that elements.
Nuclide notation can be used to present different atoms. Here we have a carbon atom with mass number on the top left and atomic number on the bottom left. Atoms 1 and 2 are isotopes of each other.
We also have a Fluorine ion with the atom’s charge on the top right.
Key Points!
-
Atomic theory
The number of protons in an atom is called the Atomic Number.
The number of protons + the number of neutrons is the Mass Number.
Protons have a mass of 1, neutrons have a mass of 1, electrons have a mass of 0.
Protons have a charge of 1+, neutrons have a charge of 0, electrons have a charge of 1-.
-
The Periodic Table
The elements in the periodic table are arranged in order of increasing atomic number. The vertical columns are called groups and the horizontal rows are called periods.
Elements in the same group have the same number of electrons in their outer energy level.
Elements in the same period have the same number of energy levels.
Group 1 are the Alkali Metals.
The transition metals are the block of elements in the middle of the periodic table.
Group 7 are the Halogens.
Group 8 are the Noble Gases.
-
Valency and Ions
Valency is an element’s capacity to combine with other elements. The valency of an element is determined by the number of electrons in an atom’s outer energy level, and so elements in the same group have the same valency.
An ion is a particle, atom or molecule with a net electrical charge.
-
Isotopes and Nuclide Notation
Isotopes are atoms that have the same number of protons but a different number of neutrons.
Nuclide notation can be used to present different atoms.