Gas Laws
By investigating properties of matter, we have been able to determine some interesting and useful relationships between them. Relationships between temperature, pressure and volume are utilised in our day-to-day lives, including within our own bodies, e.g. blood pressure and the negative pressure within our lungs, allowing the volume of air we breath in and out to vary.
We will discuss the relationships between pressure, temperature and volume.
Pressure
Pressure is force per unit area, so if you push on your desk with your hand, the pressure on the desk is the force in Newtons divided by the surface area of your hand.
P = F/A
It’s always worth remembering how to calculate the area of different shapes, including circles, squares and triangles. A question requiring pressure to be calculated may ask you to calculate the area of the surface first.
Area of a circle = Pi x radius^2
Area of a triangle = 1/2 base x height
Temperature and Kelvin
As discussed in the Heat and Temperature section of this unit, Kelvin is another measurement of Temperature. 0 Kelvin is absolute 0 and is equivalent to -273 degrees Celsius.
We will use Kelvin to show the relationships that temperature has with pressure and volume.
Pressure and Temperature
Gay-Lussac’s Law describes the relationship between pressure and temperature.
An increase in temperature increases pressure in a contained system. As temperature increases, the particles within the contained gas speed up. As these particles have increased kinetic energy, they hit off the sides of the container with more force. This causes an increased force on the surface area of the container, increasing pressure. The relationship is therefore directly proportional.
An easy way of remembering this is imagining a popcorn bag, with the bag being the container and the popcorn being the particles. As the popcorn heats up, cause increased pressure on the bag.
You can also calculate an unknown pressure or temperature in a question using the given equation.
Volume and Temperature
The relationship between volume and temperature is called Charles’ Law.
When a gas is heated in a system with flexible boundaries, such as a closed syringe in the given image, or in a popcorn bag, temperature increases the volume of the gas. When the temperature of particles in the system increase, the particles gain more energy and collide with the surface of the container with more force. This causes the gas to expand within the container due to the increased force of the fast-moving particles. The relationship between temperature and volume is therefore directly proportional.
An unknown value of temperature or volume can be calculated with the given equation.
Pressure and Volume
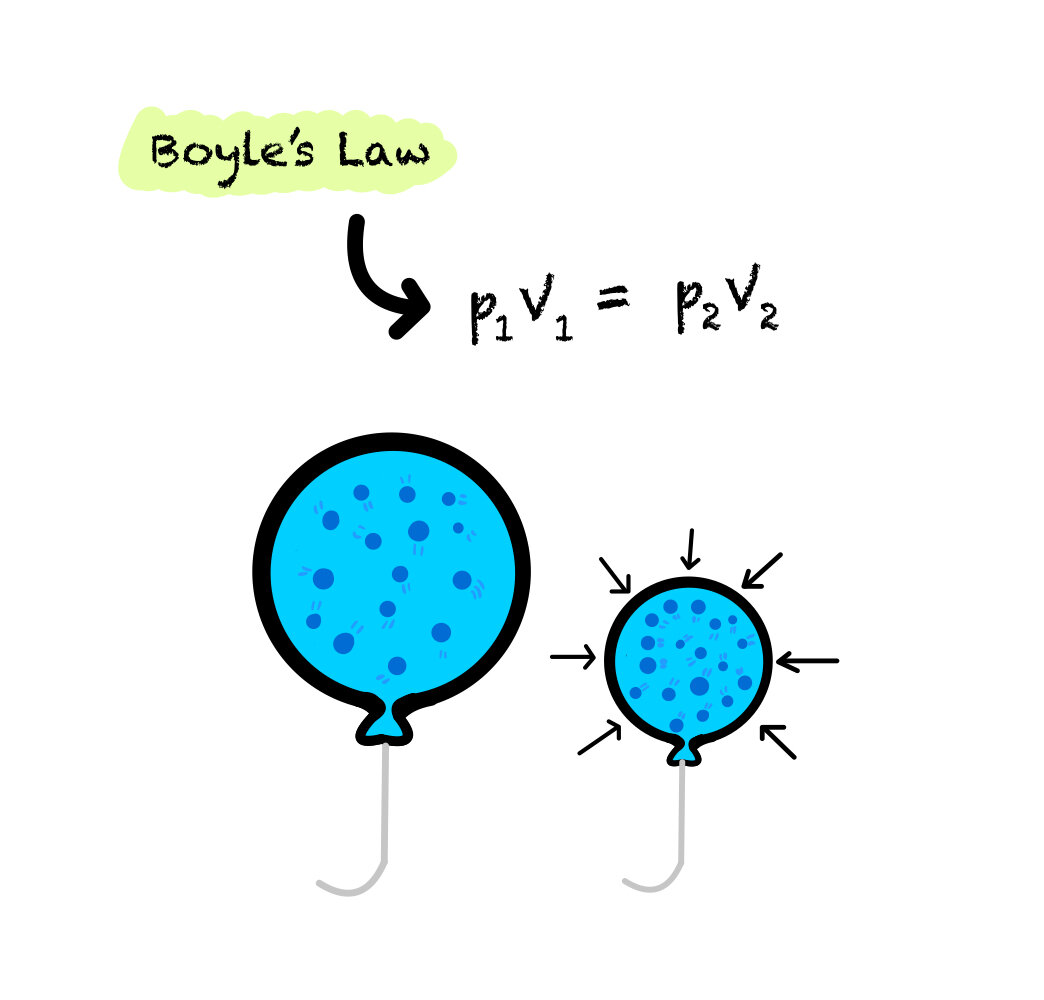
The relationship between pressure and volume is called Boyle’s Law.
When volume is decreased in a system, pressure increases. If the volume of a container is decreased, like compressing a balloon in the given image the number of particles in the gas remains the same. With the same number of particles and a decreased surface area, the particles will collide with the walls of the container more often. This will create more force per unit area, and thus increasing pressure. Therefore, this relationship is inversely proportional.
An unknown value of pressure or volume can be calculated using the given equation.
Key Points!
-
Pressure
Pressure is force per unit area.
P = F/A
-
Kelvin
0 Kelvin is absolute 0 and is equivalent to -273 degrees Celsius.
-
Temperature and Pressure
An increase in temperature increases pressure in a contained system.
This is called Guy-Lussac’s Law.
-
Temperature and Volume
As temperature increases in a system, volume increases.
This is called Charles’ Law.
-
Volume and Pressure
When volume is decreased in a system, pressure increases.
This is called Boyle’s Law.