Energy from Fuels
Have you ever looked at fire and actually questioned how fire happens?
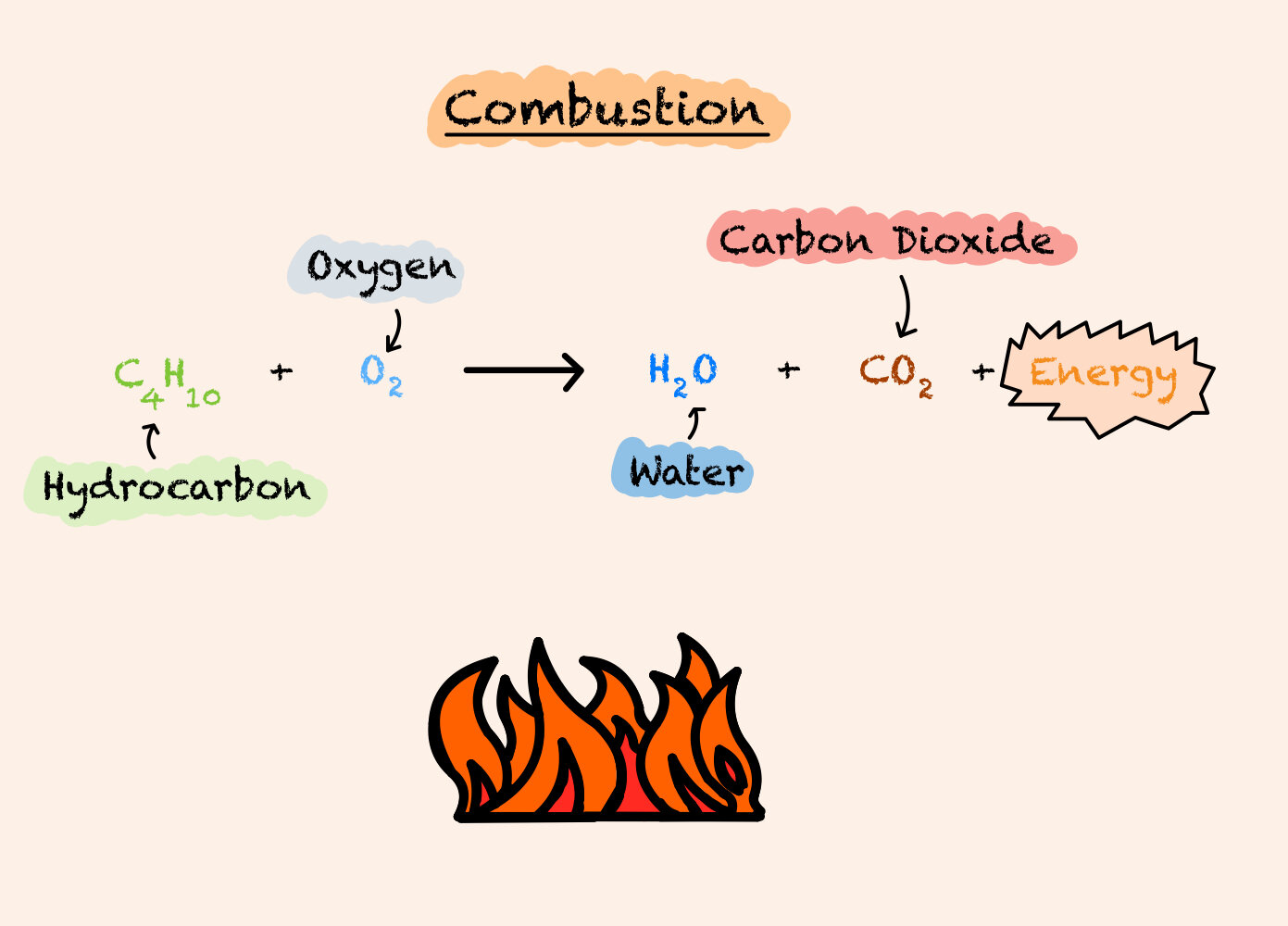
Well, question no further, because we are going to talk about combustion and how fuels give energy. When a hydrocarbon reacts with oxygen it creates energy (fire).
In this section we will also discuss specific heat capacity and how the amount of energy required to change the temperature of substances can vary between materials.
Combustion Reactions
An endothermic reaction is a chemical reaction in which the reactants absorb heat energy from their surroundings to form the products.
An exothermic reaction is a chemical reaction in which the reaction process releases heat energy to its surroundings.
A combustion reaction occurs when a substance reacts with oxygen and energy is released.
Specific Heat Capacity
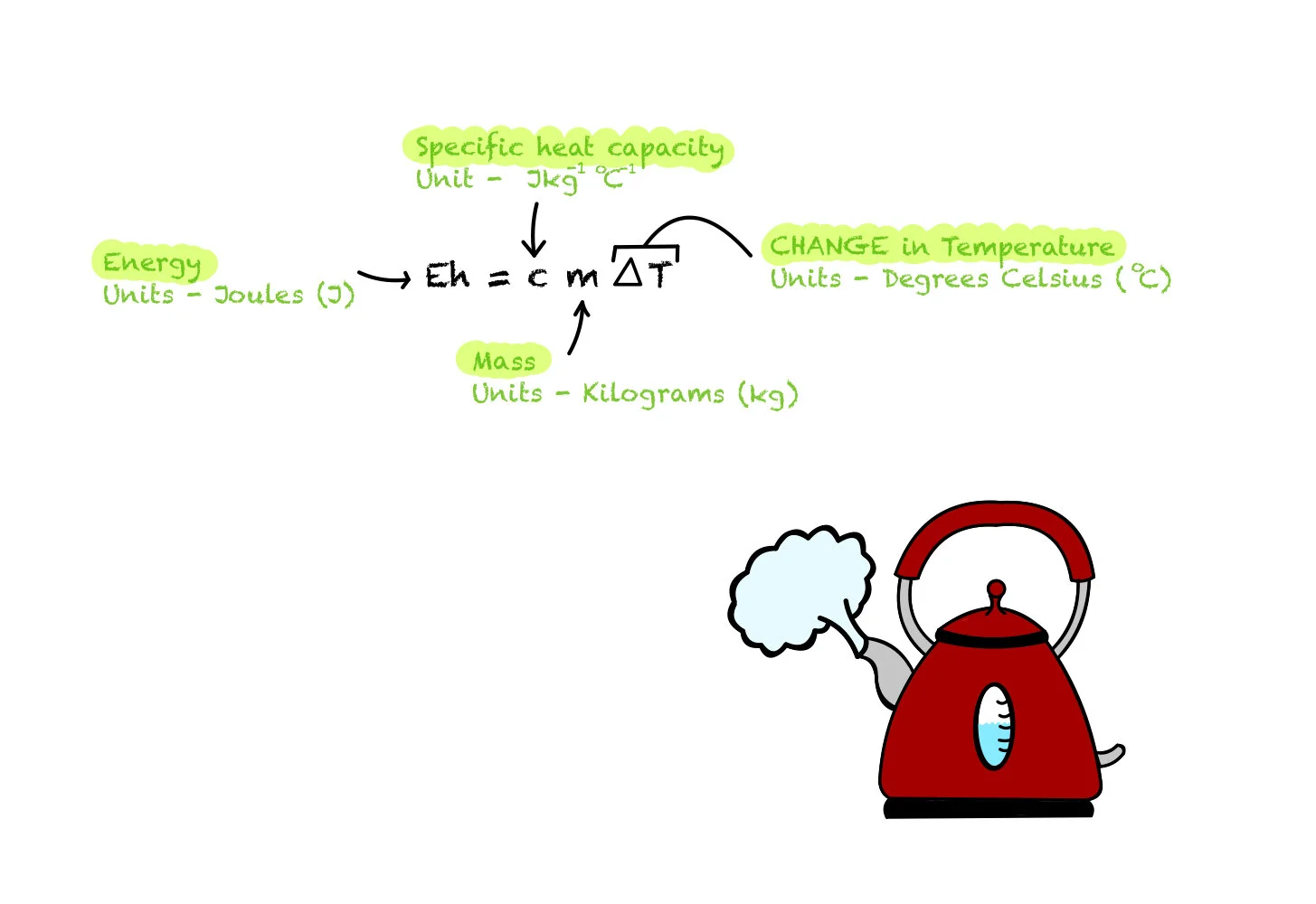
The specific heat capacity of a substance is the amount of energy required to heat that substance by 1 degree Celsius. This is an important definition to remember.
You need to use this equation and be able to rearrange it.
Remember: You need to insert the DIFFERENCE in temperature into this equation, it is a common mistake to not calculate the difference in temperature before inserting into this equation.
Key Points!
-
Combustion Reactions
An endothermic reaction is a chemical reaction in which the reactants absorb heat energy from their surroundings to form the products.
An endothermic reaction is a chemical reaction in which the reaction process releases heat energy to its surroundings.
A combustion reaction occurs when a substance reacts with oxygen and energy is released.
-
Specific Heat Capacity
The specific heat capacity of a substance is the amount of energy required to heat that substance by 1 degree Celsius. This is an important definition to remember.
Eh = cm(delta)T
You need to use this equation and be able to rearrange it.