Radiation
We can’t discuss the future of energy, medicine, smoke detectors and microwave ovens without talking about radiation. It has a fascinating past and a beyond-exciting future that is unfolding as we speak. I’m excited, are you excited?
I will discuss the theory behind this topic and explain how to use certain equations to determine how “radioactive” a substance is, as well as introduce equations to determine how much damage radiation can cause to the body. This is a valuable topic for everyone to know, so let’s start with the basics! There is a link with Chemistry in this topic so feel free to check in with “Nuclear Radiation” in National 5 Chemistry.
Atom Structure + Ionisation
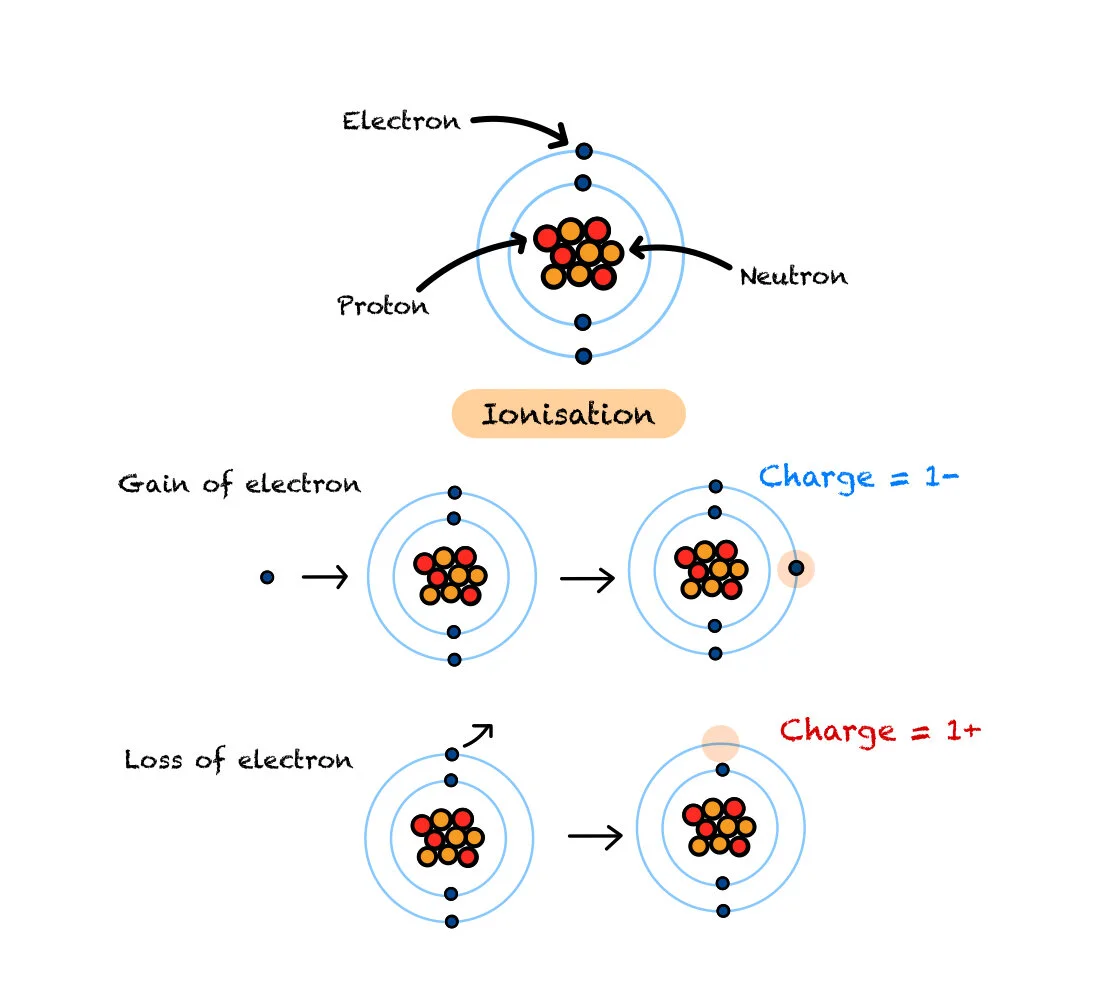
Atoms are made up of Protons, Neutrons and Electrons.
Protons have a Charge of 1+, Neutrons have a Charge of 0 and Electrons have a charge of 1-.
Protons and Neutrons both have a mass of 1 each, and Electrons have a mass of 0 (not exactly 0, but for National 5 Physics, the mass of an electron is so tiny, that it is considered 0 when compared to neutrons and protons).
Ionisation is when a neutral atom (balanced number of protons and electrons) loses or gains an electron, which creates an Ion. Electrons can be lost or gained due to IONISING RADIATION.
If ionising radiation makes contact with an atom, it can cause it to ionise (gain or lose an electron), which can make it unstable.
Radiation types
There are 3 types of radiation that you need to be aware of:
ALPHA PARTICLE
This is a helium nucleus (two protons and two neutrons). This has a charge of 2+ as it contains two protons. These are the most ionising (damaging) and also the slowest. They can be blocked easiest with just paper.
BETA PARTICLE
A Beta particle is a FAST MOVING ELECTRON. These have a charge of 1- and a mass of 0. These are the second most ionising.
GAMMA WAVES
Gamma waves are part of the Electromagnetic Spectrum. They travel a the speed of light (3x10^8m/s) so travel the fastest, and are the least ionising.
Ionising effect and penetration
If you are to remember anything from this this section, remember the ionising and penetration potentials of Alpha, Beta and Gamma radiation.
Alpha particles are the least penetrative but the most ionising (damaging)
Beta particles are the second most penetrative and the second most ionising.
Gamma particles are the most penetrative but the least ionising.
Key Points!
-
Ionising Radiation
Understand the structure of the atom
Ionising radiation can cause an atom to gain or lose an electron.
Ionsising radiation can be dangerous.
-
Types of Radiation
Know the nature, charge and mass of Alpha, Beta and Gamma radiation.
-
Ionisation and Penetration
Know how ionising and penetrative Alpha, Beta and Gamma radiation are in order.