Nuclear Chemistry
We can’t discuss the future of energy, medicine, smoke detectors and microwave ovens without talking about radiation. It has a fascinating past and a beyond-exciting future that is unfolding as we speak. I’m excited, are you excited?
Radiation
Ionisation is when a neutral atom (balanced number of protons and electrons) loses or gains an electron, which creates an Ion. Electrons can be lost or gained due to IONISING RADIATION.
If ionising radiation makes contact with an atom, it can cause it to ionise (gain or lose an electron), which can make it unstable.
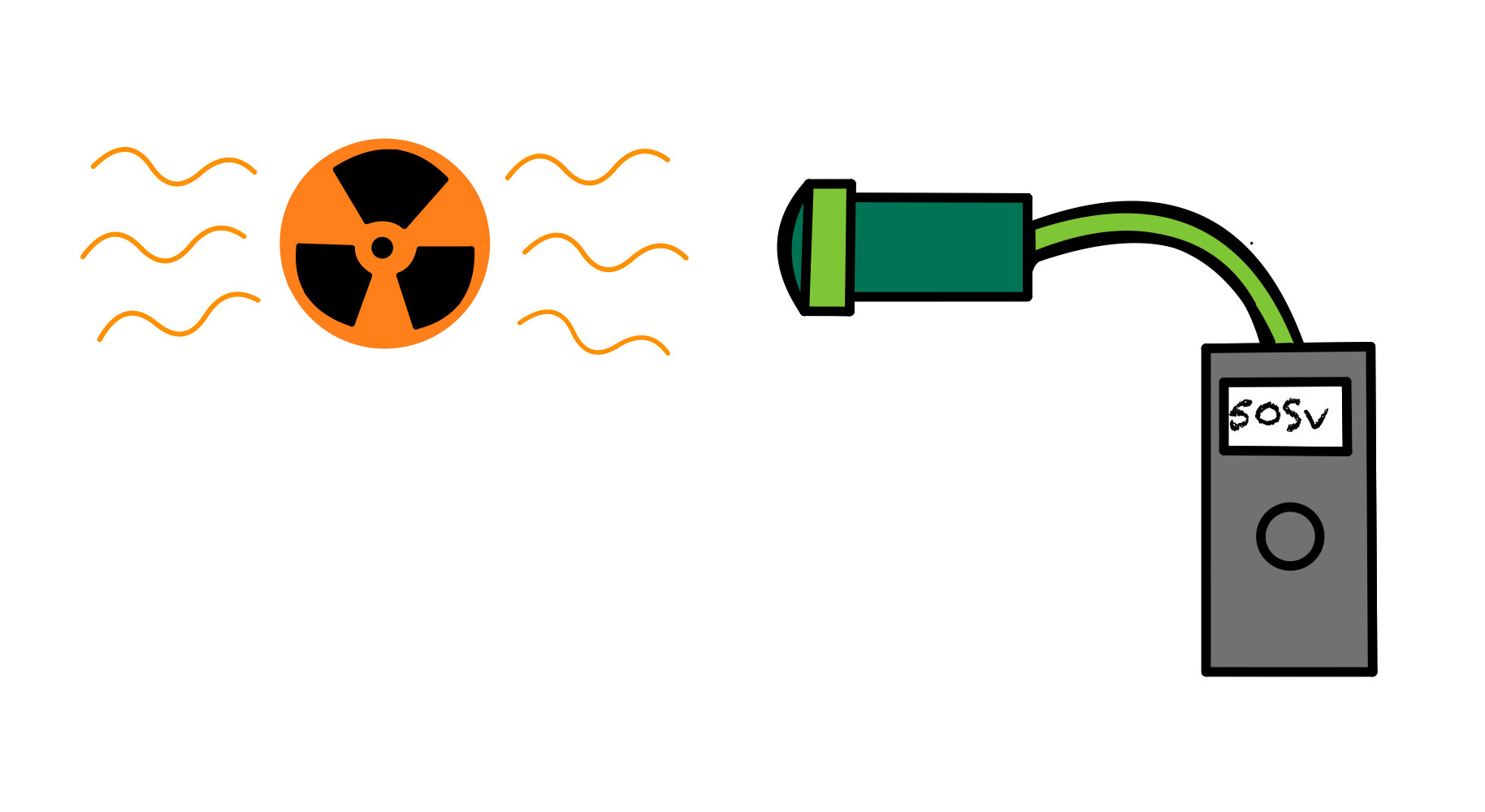
A Geiger-Muller tube can be used to detect ionising radiation.
Ionising radiation can damage DNA and cause burns and cancers.
Alpha, Beta, Gamma
There are 3 types of radiation that you need to be aware of:
ALPHA PARTICLE
This is a helium nucleus (two protons and two neutrons). This has a charge of 2+ as it contains two protons. These are the most ionising (damaging) and also the slowest. They can be blocked easiest with just paper.
BETA PARTICLE
A Beta particle is a FAST MOVING ELECTRON. These have a charge of 1- and a mass of 0. These are the second most ionising.
GAMMA WAVES
Gamma waves are part of the Electromagnetic Spectrum. They travel a the speed of light (3x10^8m/s) so travel the fastest, and are the least ionising.
Ionising effect and penetration
If you are to remember anything from this this section, remember the ionising and penetration potentials of alpha, beta and gamma radiation.
Alpha particles are the least penetrative but the most ionising (damaging)
Beta particles are the second most penetrative and the second most ionising.
Gamma particles are the most penetrative but the least ionising.
Nuclear Equations
Note the given nuclide notation for alpha particles, beta particles and protons.
You need to be able to identify different emissions from equations like the two provided.
An emission of an alpha particle leads to a decrease in atomic number by 2 and a decrease in mass number by 4.
Beta particles are emitted when a neutron splits into a proton and an electron. The electron is emitted and the atomic number increases by 1. Mass number stays the same.
Half-Life
As a radioactive substance ages, its activity (no. decays/time) decreases. Each radioactive substance has a very specific time in which its activity is halved. For example, one isotope’s activity may take 3 years to reduce by half. The time that a radioactive substance takes for its activity to reduce by half is called half-life.
Most half-life questions are problem solving, and the key to studying them is practice, practice, practice. These are very common questions in exams, and it can be easy to drop marks on these calculations.
Uses of Radiation
Radiation has many uses, including in the production of energy, as well as in the medical field.
In the medical field radiation is used in the form of X-rays for imaging/diagnosing patients. X-rays can also be used therapeutically, helping in surgical procedures. Gamma radiation is utilised in radiotherapy for certain cancers and also used to sterilise equipment for surgeries.
Knowledge of radiation has allowed us to determine the ages of old carbon-based compounds. This is called carbon-dating and by determining the half-life of a carbon-based compound we are able to calculate the age of the substance. Radiation is therefore used in archaeology.
Radiation can also be used for energy production, space exploration and in smoke detectors.
Key Points!
-
Radiation
Electrons can be lost or gained due to IONISING RADIATION.
If ionising radiation makes contact with an atom, it can cause it to ionise (gain or lose an electron), which can make it unstable.
A Geiger-Muller tube can be used to detect ionising radiation.
Ionising radiation can damage DNA and cause burns and cancers.
-
Types of Radiation
There are 3 types of radiation that you need to be aware of:
ALPHA PARTICLE
- This is a helium nucleus (two protons and two electrons). This has a charge of 2+ as it contains two protons. These are the most ionising (damaging) and also the slowest. They can be blocked easiest with just paper.
BETA PARTICLE
- A Beta particle is a FAST MOVING ELECTRON. These have a charge of 1- and a mass of 0. These are the second most ionising.
GAMMA WAVES
- Gamma waves are part of the Electromagnetic Spectrum. They travel a the speed of light (3x10^8m/s) so travel the fastest, and are the least ionising.
-
Ionising Effect and Penetration
Alpha particles are the least penetrative but the most ionising (damaging)
Beta particles are the second most penetrative and the second most ionising.
Gamma particles are the most penetrative but the least ionising.
-
Nuclear Equations
Be able to write the nuclear equations when an alpha or beta particle is emitted from an atom.
Know the nuclide notation for different radioactive particles.
-
Half-Life
As a radioactive substance ages, its activity (no. decays/time) decreases. Each radioactive substance has a very specific time in which its activity is halved. For example, one isotope’s activity may take 3 years to reduce by half. The time that a radioactive substance takes for its activity to reduce by half is called half-life.
Be able to answer half-life problem solving questions.
-
Radiation Uses
Know the applications of radiation including in the medicine field and energy production.