Metals
Metals is a chunky section of National 5 Chemistry which can be quite hard to get your head around. Here, I will break down the fundamentals of this topic and highlight the points to remember.
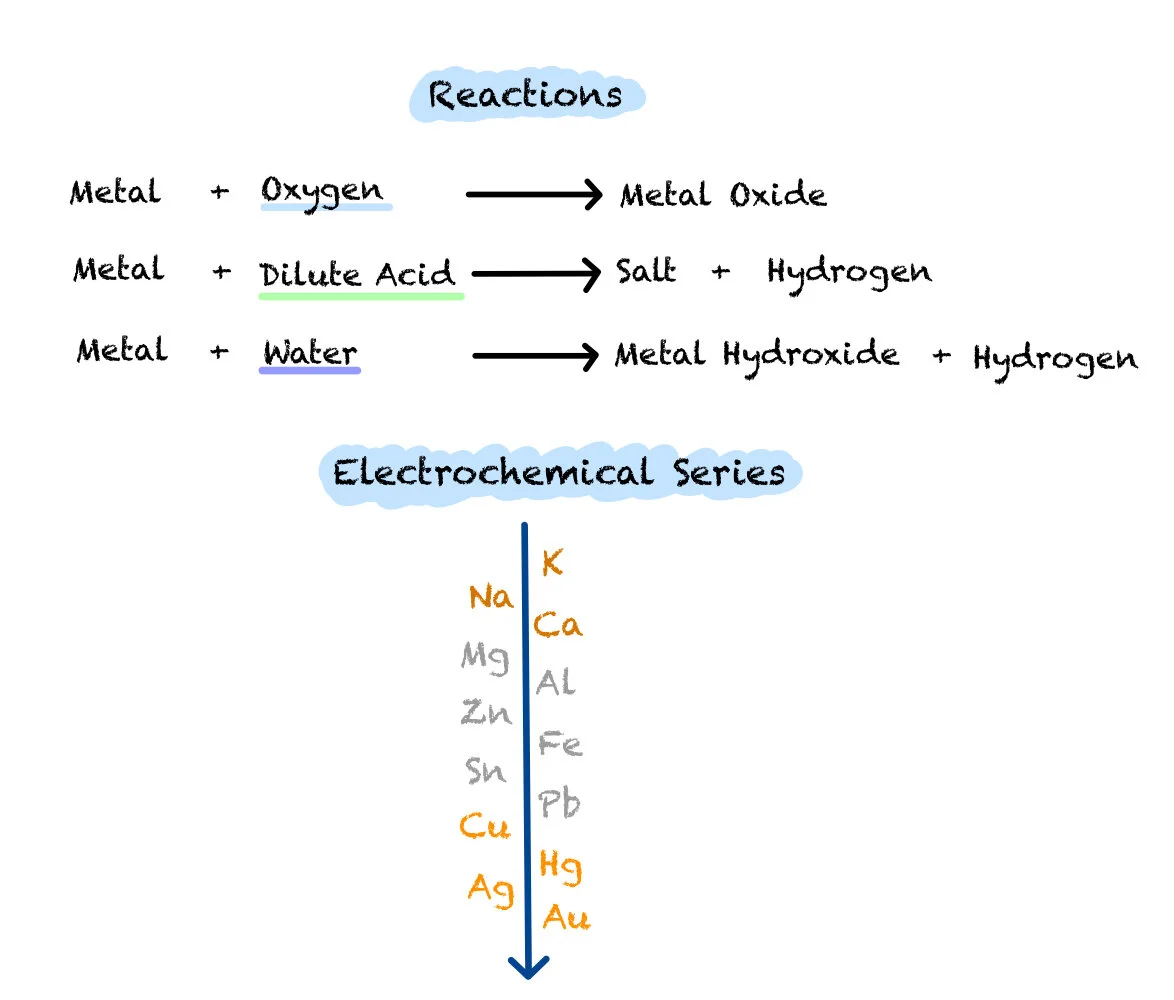
Metals are put in order of their reactivity in a list called the Electrochemical Series. This can be found in your data booklet and this series determines various properties of different metals.
Metallic Bonding
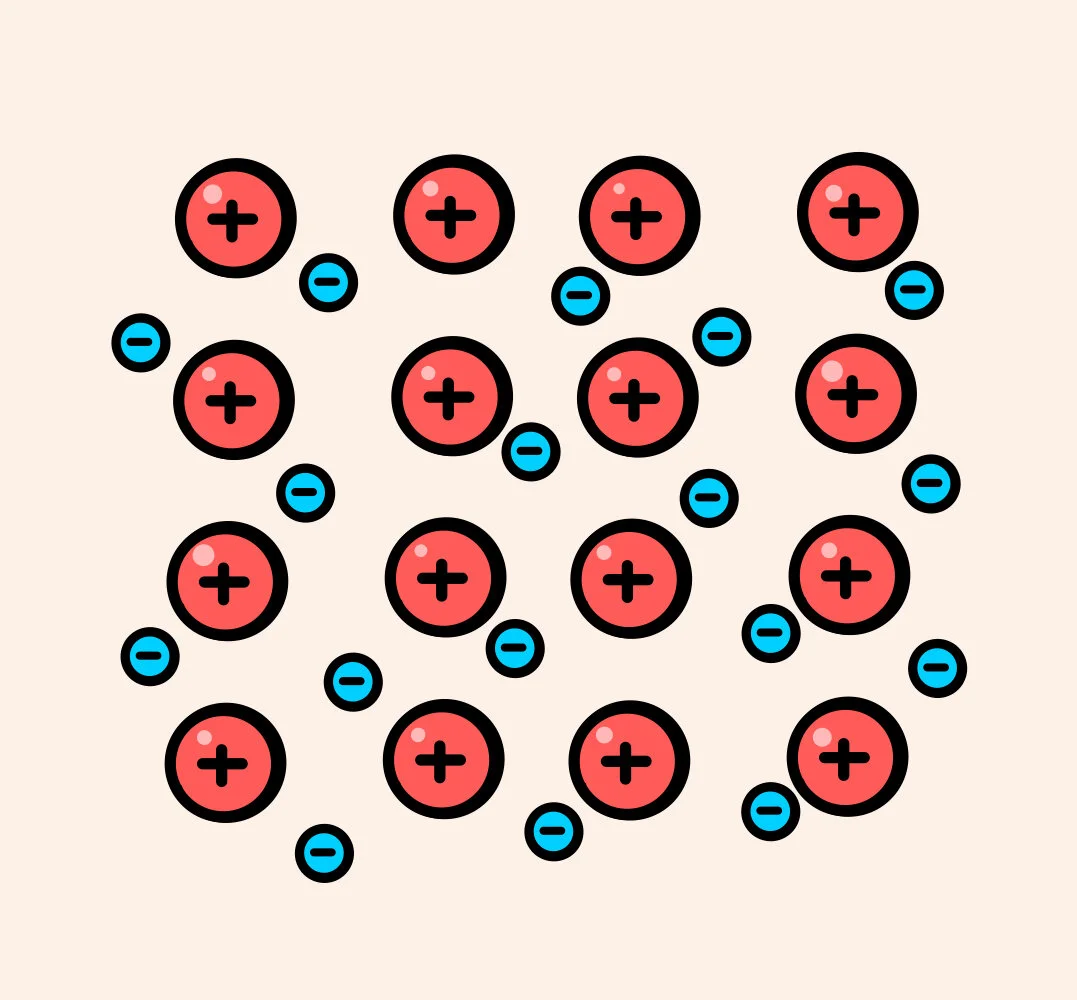
Metallic bonding is, you guessed it, the bonding between metals.
This is where many positive ions bind together, surrounded by a sea of delocalised electrons.
This sea of delocalised electrons are free to move, meaning that metals conduct electricity.
The question: “How can metals conduct electricity,” is very common in exams, which makes it important to remember that delocalised electrons are free to move in metallic bonding.
Reactions with Metals
Metals can undergo reactions with oxygen, acids and water.
Metal + Oxygen ———> Metal Oxide
Metal + Dilute Acids ———> Salt + Hydrogen
Metal + Water ———> Metal Hydroxide + Hydrogen
The electrochemical series puts metals in order of how reactive they are. Only the most reactive metals react with water to create a metal hydroxide and hydrogen. More, but not all of the metals on the electrochemical series can react with dilute acids. Most metals on the series react with Oxygen to create a metal oxide.
Extracting Metals
Metal need to be extracted from ores for various uses. There are few different ways that we can extract metals from their ores.
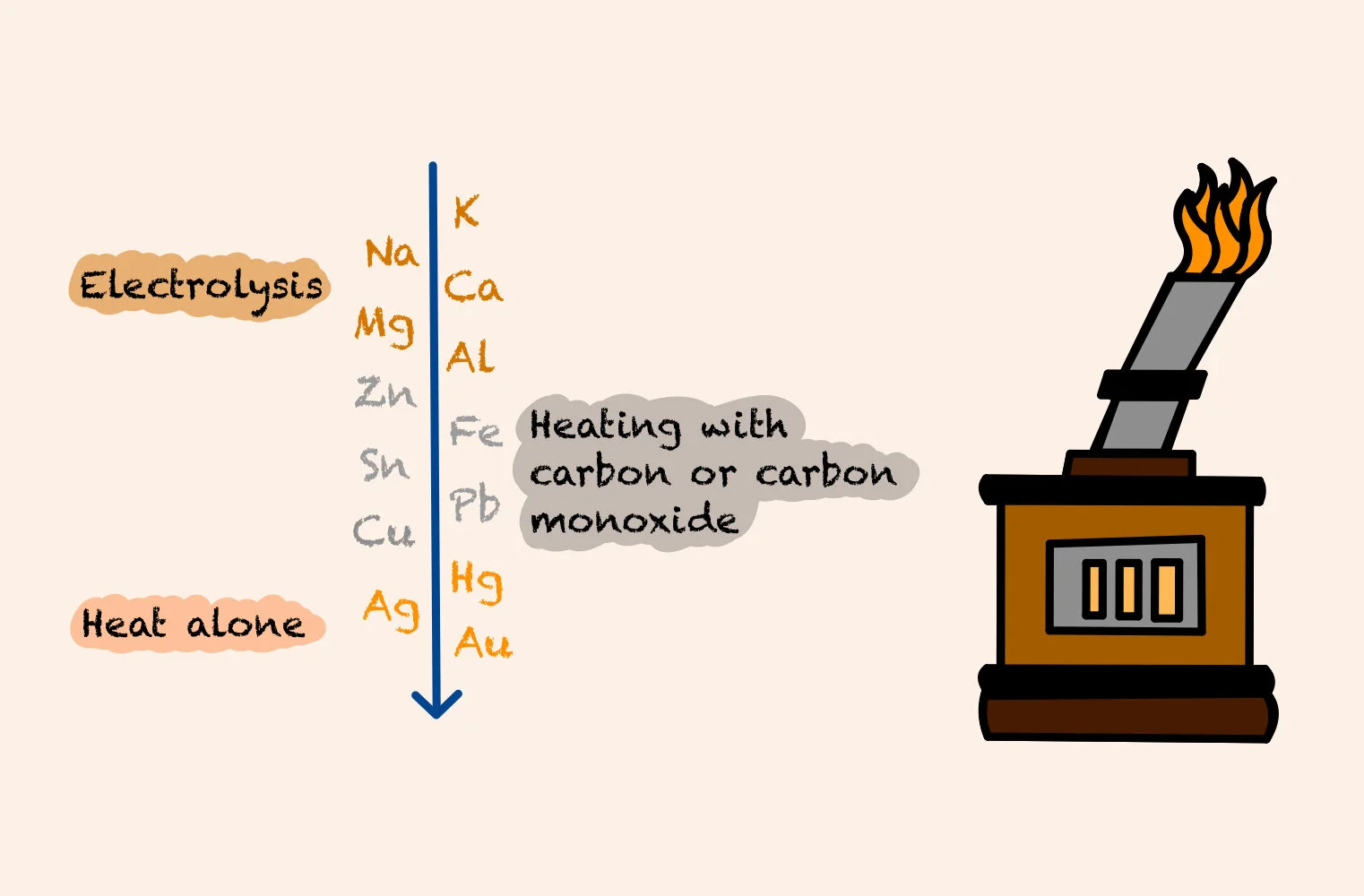
More reactive metals (those high up in the electrochemical series) need to be extracted from their ores using electrolysis. Electrolysis is a process used to break down ionic substances by passing a direct electric current through the substance. This causes chemical reactions at the electrodes and decomposition of materials.
Those in the middle of the electrochemical series need to be extracted by heating with carbon or carbon monoxide.
Heat alone can be used to extract metals that aren’t very reactive, such as gold.
Electrochemical Cells
Electrical energy travels from metals high up in the electrochemical series to ones lower down in the electrochemical series within electrochemical cells.
A simple cell can be made by placing two different metals in an electrolyte solution.
Another type of cell can be made by creating two half-cells through placing metals in solutions containing that metal’s ions. These two half calls can be connected using an ion bridge to complete the circuit.
Redox Reactions
Redox reactions describe the transfer of electrons between atoms.
But let’s first break them down into oxidation reactions and reduction reaction.
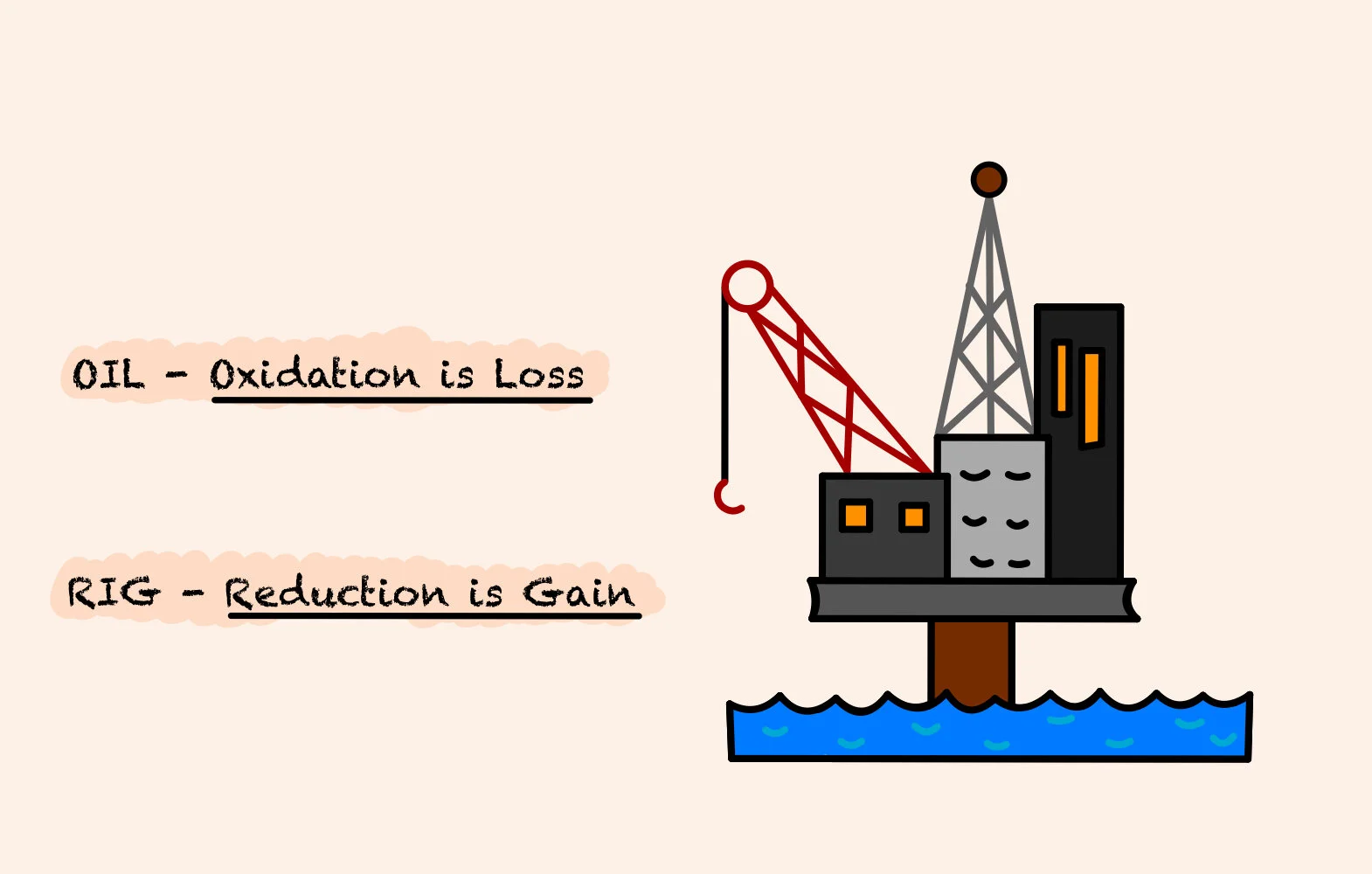
OIL RIG - Oxidation is Loss (of electrons), Reduction is Gain (of electrons).
Therefore, when an atom loses electrons in a reaction, this is called oxidation and the atom is oxidised.
When an atom gains an electron in a reaction, this is called reduction and the atom is reduced.
Reducing agents are compounds that are used in reactions to reduce (give an electron) to another molecule, and in turn are oxidised themselves.
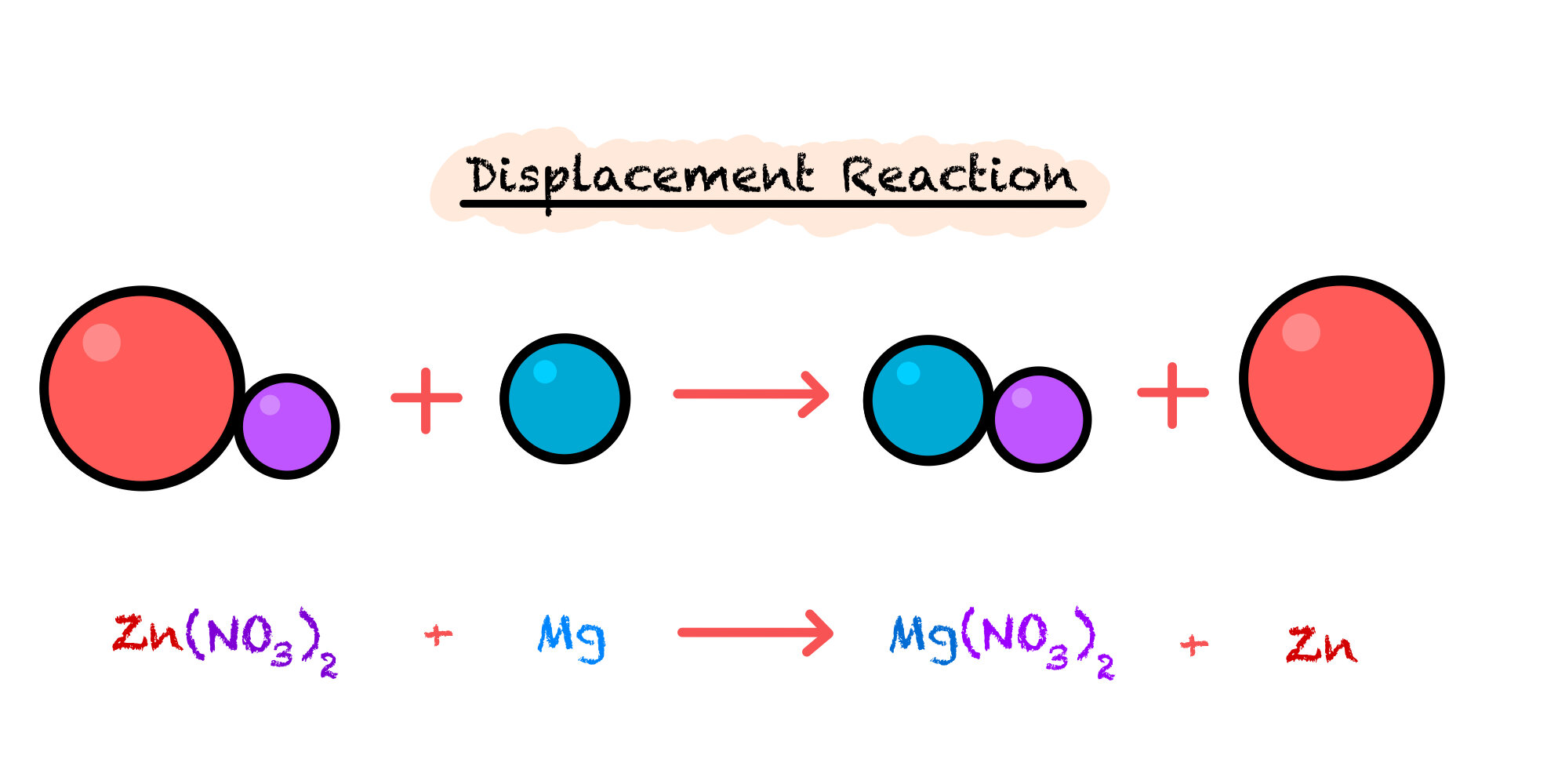
Displacement Reactions
Displacement reactions occur when a compound containing a metal of the electrochemical series mixes with a compound containing another metal lower down on the electrochemical series. The compound with the more reactive metal atoms will displace those of the less reactive metal.
Key Points!
-
Metallic Bonding
This is where many positive ions bind together, surrounded by a sea of delocalised electrons.
This sea of delocalised electrons are free to move, meaning that metals conduct electricity.
-
Reactions with metals
Metals can undergo reactions with oxygen, acids and water.
Metal + Oxygen ———> Metal Oxide
Metal + Dilute Acids ———> Salt + Hydrogen
Metal + Water ———> Metal Hydroxide + Hydrogen
The electrochemical series puts metals in order of how reactive they are. Only the most reactive metals react with water to create a metal hydroxide and hydrogen. More, but not all of the metals on the electrochemical series can react with dilute acids. Most metals on the series react with Oxygen to create a metal oxide.
-
Extracting Metals
More reactive metals (those high up in the electrochemical series) need to be extracted from their ores using electrolysis. Electrolysis is a process used to break down ionic substances by passing a direct electric current through the substance. This causes chemical reactions at the electrodes and decomposition of materials.
Those in the middle of the electrochemical series need to be extracted by heating with carbon or carbon monoxide.
Heat alone can be used to extract metals that aren’t very reactive, such as gold.
-
Electrochemical Cells
Electrical energy travels from metals high up in the electrochemical series to ones lower down in the electrochemical series within electrochemical cells.
A simple cell can be made by placing two different metals in an electrolyte solution.
Another type of cell can be made by creating two half-cells through placing metals in solutions containing that metal’s ions. These two half calls can be connected using an ion bridge to complete the circuit.
-
Redox Reactions
When an atom loses electrons in a reaction, this is called oxidation and the atom is oxidised.
When an atom gains an electron in a reaction, this is called reduction and the atom is reduced.
Reducing agents are compounds that are used in reactions to reduce (give an electron) to another molecule, and in turn are oxidised themselves.
-
Displacement Reactions
Displacement reactions occur when a compound containing a metal of the electrochemical series mixes with a compound containing another metal lower down on the electrochemical series. The compound with the more reactive metal atoms will displace those of the less reactive metal.