Chemical Analysis
We need to discuss the various reactions, methods and analysis methods if we want to be a true mad scientist!
Understanding the various types of data in chemistry is vital for future studies you may do in science. These are all very common questions in exams that people trip up on as they forget that this is all part of the course, so be sure to test yourself on precipitation reactions and how to collect gases!
Quantitative vs Qualitative
Quantitative analysis and data is information about quantities and numbers. Therefore, quantitative analysis will answer the questions such as: “what?” or “how many?” This can be used to measure the amount of a chemical in a sample. For example, measuring the concentration of a chemical.
Qualitative analysis is more descriptive and is often described in words. Qualitative data describes qualities or characteristics and can be used to determine then presence of a substance in a sample. For example, flame tests can be used to determine the presence of a chemical.
Precipitation Reactions
Insoluble - Cannot be dissolved
Solution - A liquid in which a smaller component (solute) is distributed uniformly within a larger component (solvent).
Solute - A minor component in a solution, dissolved in a solvent.
Solvent - A substance that dissolves other substances.
A precipitation reaction is a chemical reaction where two soluble salts in an aqueous solution combine, leading to an insoluble salt being formed as a product. This insoluble product formed is called a precipitate.
Gas Tests
You can test for hydrogen gas by lighting a splint and inserting it into a test tube with hydrogen gas suspected to be in it. If hydrogen is present, it will burn with a pop.
To test for oxygen, insert a glowing splint into a test tube with oxygen suspected to be in it. If oxygen is present it will relight the splint.
To test for carbon dioxide, bubble gas through lime water. If carbon dioxide is present it will turn lime water cloudy.
Collecting Gases
The given diagrams show how to collect gases of different densities.
Insoluble gases can be collected using method A.
Soluble gases, less dense than air, can be collected using method B.
Soluble gases, more dense than air, can be collected using method C.
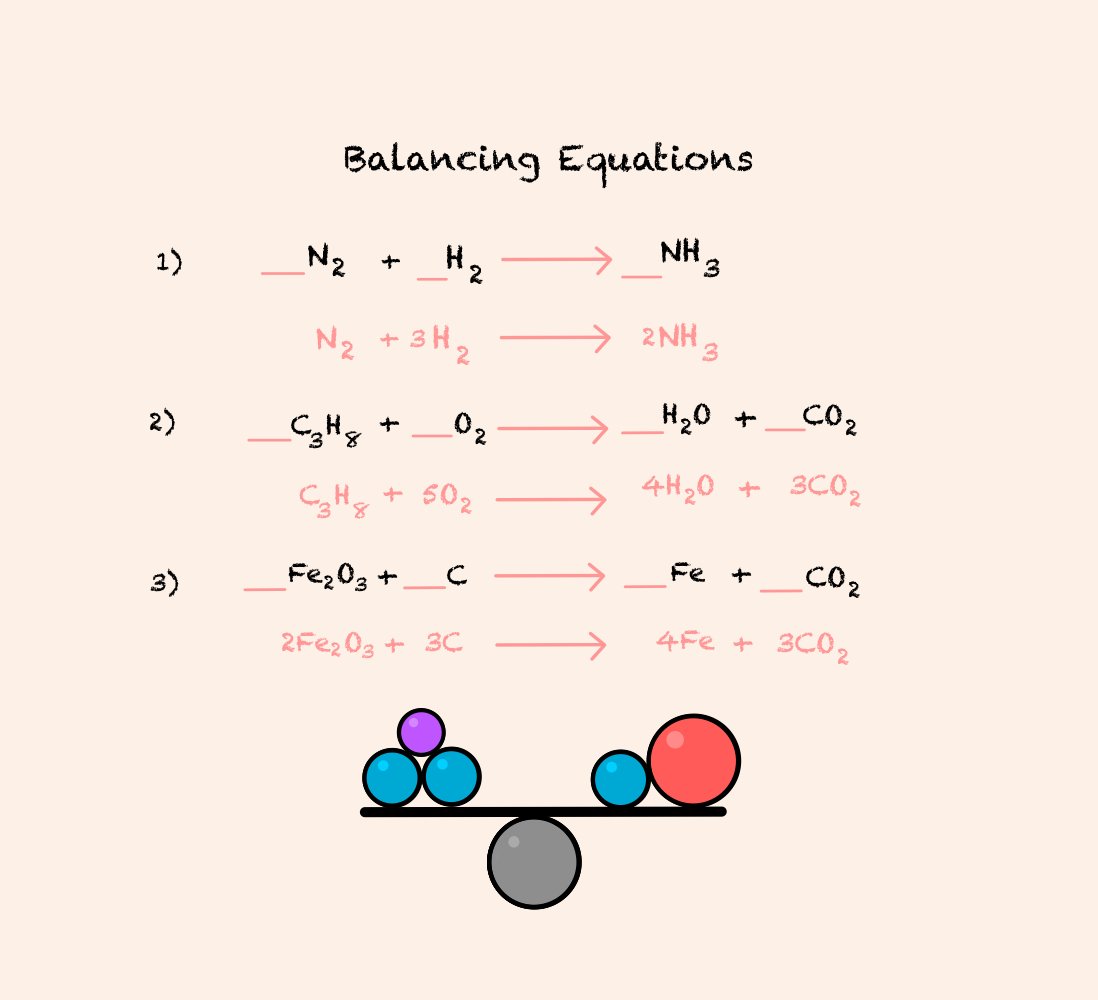
Balancing Equations
Balancing equations is best learned through practice. To balance equations, your job is to make sure that the same number of each element are on each side of the equation.
I have demonstrated 3 examples of balancing equations and, as you can see, it’s all about multiplication.
Do as many practice questions as you can, because it really is all about trial and error with these questions.
Key Points!
-
Quantitative vs Qualitative
Quantitative analysis and data is information about quantities and numbers. Therefore, quantitative analysis will answer the questions such as: “what?” or “how many?” This can be used to measure the amount of a chemical in a sample. For example, measuring the concentration of a chemical.
Qualitative analysis is more descriptive and is often described in words. Qualitative data describes qualities or characteristics and can be used to determine then presence of a substance in a sample. For example, flame tests can be used to determine the presence of a chemical.
-
Precipitation Reactions
A precipitation reaction is a chemical reaction where two soluble salts in an aqueous solution combine, leading to an insoluble salt being formed as a product. This insoluble product formed is called a precipitate.
-
Gas Tests
You can test for hydrogen gas by lighting a splint and inserting it into a test tube with hydrogen gas suspected to be in it. If hydrogen is present, it will burn with a pop.
To test for oxygen, insert a glowing splint into a test tube with oxygen suspected to be in it. If oxygen is present it will relight the splint.
To test for carbon dioxide, bubble gas through lime water. If carbon dioxide is present it will turn lime water cloudy.
-
Collecting Gases
Know how to collect insoluble gases.
Know how to collect soluble gases less dense than air.
Know how to collect soluble gases more dense than air.
-
Balancing Equations
Know how to and practice balancing equations.